You've heard that 'one bad apple spoils the whole
barrel', right? It's true. Bruised, damaged, or overripe fruit gives off a
hormone that accelerates the ripening of the other fruit -- ETHYLENE.

What is
ETHYLENE ?
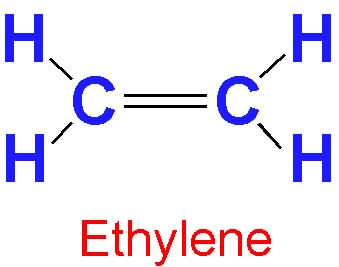
Ethylene (C2H4, also known as ethene) is a
gaseous organic compound that is the simplest of the alkene chemical structures
(alkenes contain a carbon-carbon double bond). Ethylene is the most
commercially produced organic compound in the world and is used in many industrial
applications.
Plant tissues
communicate by means of hormones. Hormones are chemicals that are produced in
one location that have an effect on cells in a different
location. Ethylene is the hormone that will cause a wide range of effects
in plants, such as fruit ripening, loss of chlorophyll, abortion of plant
parts, stem shortening, abscission of plant parts, and epinasty (bending of
stems) which depending on the age of the plant and how sensitive the
plant is to ethylene. Ethylene can be either good or bad, depending on what
merchandise you work with. It is used in a positive manner in fruit ripening,
for instance. It can also cause damage in crops. Examples of damage might
include yellowing of vegetables, bud damage in dormant nursery stock, or abscission
in ornamentals (leaves, flowers drop off). Most plant hormones are
transported through the plant vascular system, but some, like ethylene, are
released into the gaseous phase, or air.
Where does it come from?
Ethylene is produced and
released by rapidly-growing plant tissues. It is released by the growing tips
of roots, flowers, damaged tissue, and ripening fruit. The hormone has multiple
effects on plants. One is fruit ripening. When fruit ripens, the starch in the
fleshy part of the fruit is converted to sugar. The sweeter fruit is more
attractive to animals, so they will eat it and disperse the seeds. Ethylene
initiates the reaction in which the starch is converted into sugar.
Commercial Use of Ethylene to Ripen Fruit
Climacteric fruits are frequently harvested at a
physiological stage that is considered ‘commercial maturity’, typically in a
hard green but mature stage just before ripening has initiated. Examples
include bananas, mangoes, tomatoes and avocados. This enables the fruit
to be harvested, cooled, stored and transported significant distances to where
it will be marketed and consumed.
Ripening can then be conducted under controlled
conditions of temperature, relative humidity and ethylene to achieve uniform
appearance and quality of ripe fruit. Fruit is placed into specially
constructed ripening rooms and brought to optimum ripening temperature and
humidity. Ethylene is then raised to a prescribed concentration using
either a "catalytic generator" that makes ethylene gas from liquid
ethanol or from commercially available gas supplies. Forced-air cooling
systems ensure that fruit are uniformly exposed to the room ethylene
concentration. When fruit are exposed to ethylene under these controlled
conditions they will initiate their respiratory climacteric pattern and ripen
at a relatively uniform rate. Conditions and duration can be varied to
suit customer specifications for stage of ripening and colour development.
However, concerns are periodically raised in
mass media about fruit being ‘gassed’, implying that this confers some residual
food safety risk from the ethylene gas and that the fruit has been somehow
rendered ‘unnatural’. The public should understand that the commercial
use of ethylene for fruit ripening is at a low concentration and simply
initiates the respiratory climacteric. The ethylene used commercially has
the same molecular structure. By the time the ethylene-treated fruit
reaches the consumer the climacteric may have started, there is no trace of
applied ethylene gas, any ethylene emitted by the fruit is generated by the
fruit itself and is of a much greater concentration. Therefore, there
are no food safety issues associated with the consumption of climacteric fruit.



No comments:
Post a Comment